
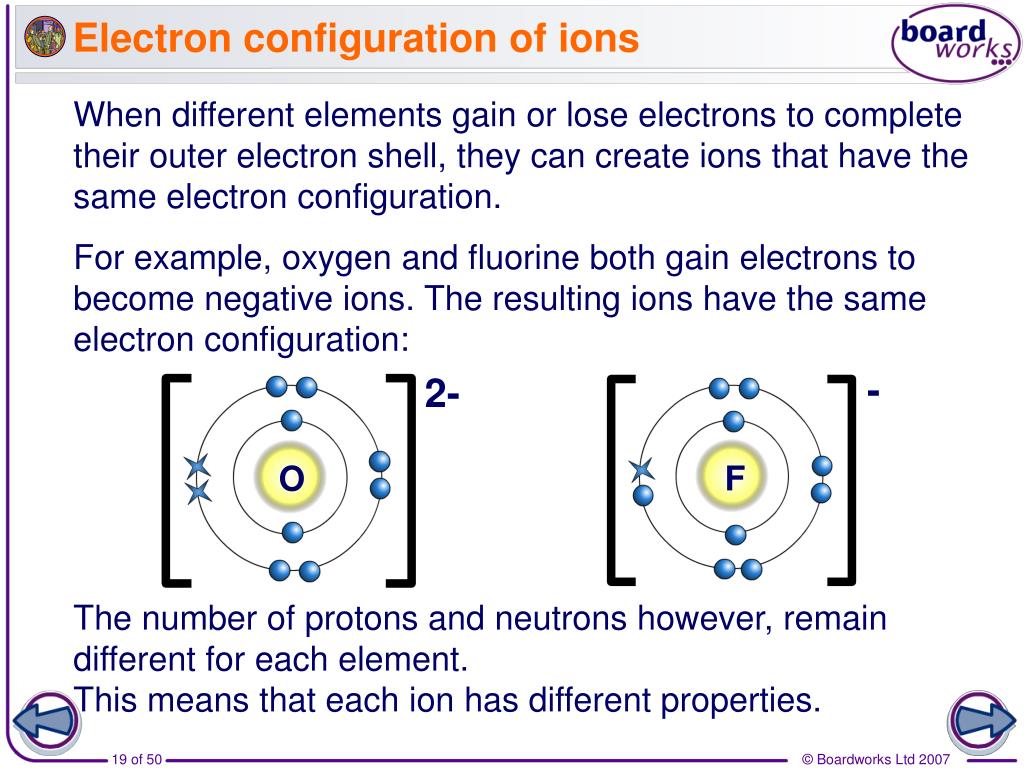
Therefore, the isotopes of an element have the same number of protons but differ in the number of neutrons. Several atomic structures of an element can exist, which differ in the total number of nucleons.These variants of elements having a different nucleon number (also known as the mass number) are called isotopes of the element. Neutrons - are electrically neutral particles and carry no charge.Electron - are negatively charged subatomic particles.Protons - are positively charged subatomic particles.Later, the scientists discovered particles inside the atom that proved, the atoms are divisible.Nothing about the structure of atom was appropriately explained.The theory was unable to explain the existence of isotopes.Atoms can neither be created nor be destroyed but can be transformed from one form to another.Atoms undergo rearrangement during a chemical reaction.Each atom has its own constant mass that varies from element to element.Specific elements have only one type of atoms in them.The following are the postulates of his theory: Dalton’s Atomic Theoryĭalton proposed that every matter is composed of atoms that are indivisible and indestructible. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. Electron charge = e = 4.8032x10 -10 esu = 1.The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it.Radiation Density Constant = a = 7.56591x10 -15 erg cm -3 K -4.Stefan-Boltzmann Constant = &sigma = 5.67051x10 -5 erg cm -2 K -4 s -1.Gravitational Constant = G = 6.67259x10 -8 cm 3 gram -1 s -2.Solar Surface Effective Temperature = 5770 K.1 Solar Luminosity = 3.826x10 33 ergs/s = 3.826x10 26 Joules/s = 3.826x10 26 Watts Unit of energy in SI system is (1) Erg (2) Calorie (3) Joule (4) Electron volt Practice questions, MCQs.1 eV = 1.602177x10 -12 erg = 1.602177x10 -19 Joule If e is the charge of an electron in esu m is the mass in grams and v the voltage 'h' is the planck constant in erg sec then the wavelength of the electron.Area of a Sphere = 41252.96124 square degrees = 4 pi Steradians.
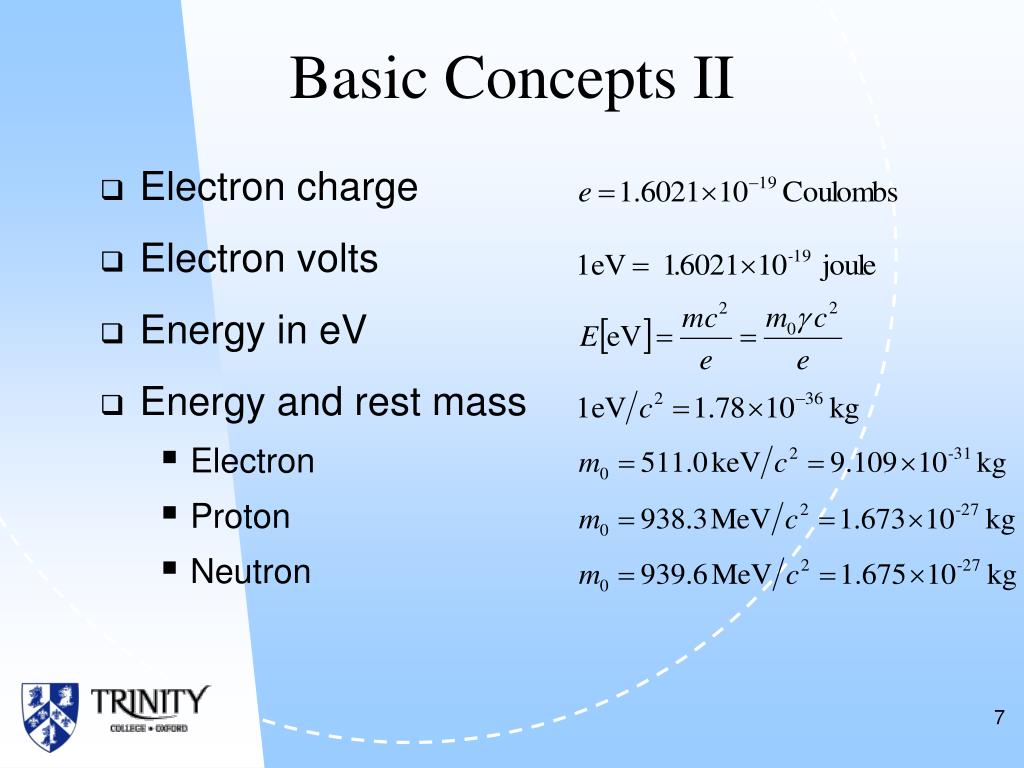
Classical Electron Radius = 2.81794x10 -15 m = e 2/ m e c 2.Unit Conversions Physical & Astronomical Constants


 0 kommentar(er)
0 kommentar(er)
